


This research group is investigating new implant materials in 3D cell culture models and the dynamic cultivation of autologous (endothelial) cell systems in special pulse wave bioreactors for a vascular graft. Microstructured scaffolds and new bioinks are designed using 3D printing. In addition, lateral flow tests are developed, e.g., after kidney transplantation, as well as optogenetically active cell systems for drug release in the inner ear (Hearing4All). KI-based indicators from complex databases support, among other things, health care research projects (MinDial, focus: patients with kidney disease).
Development of a vascular bioprosthesis
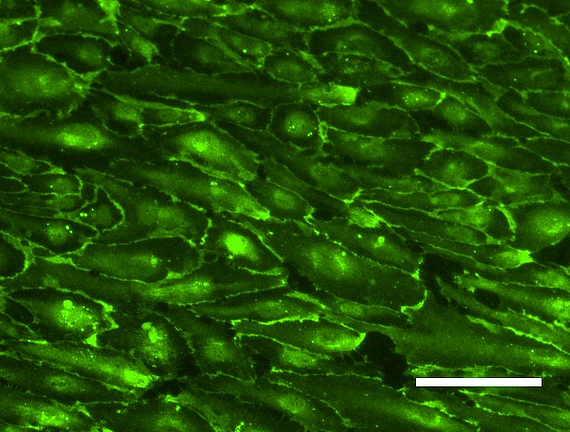
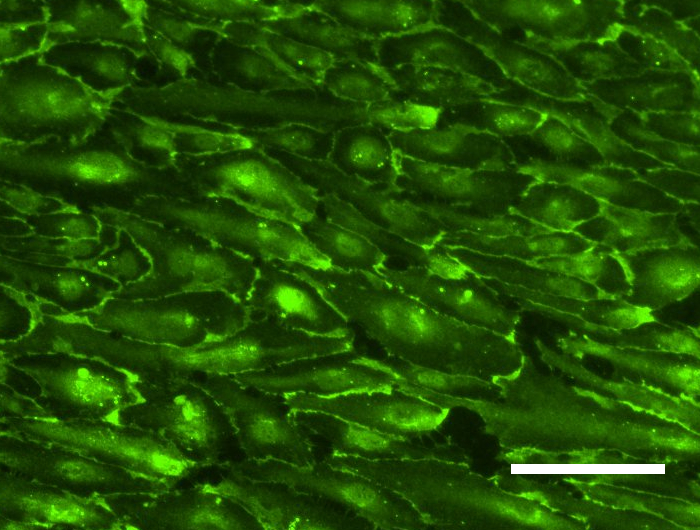

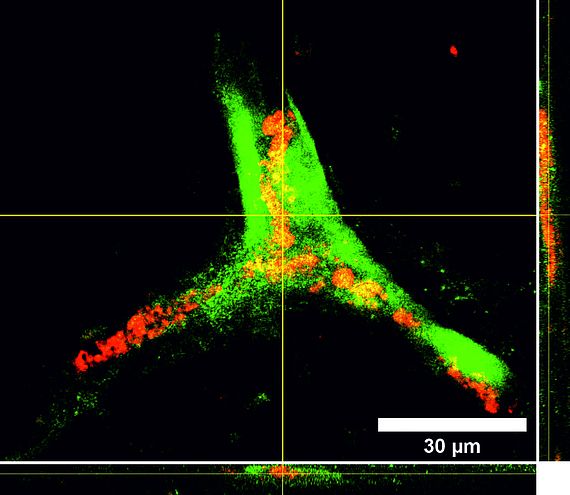
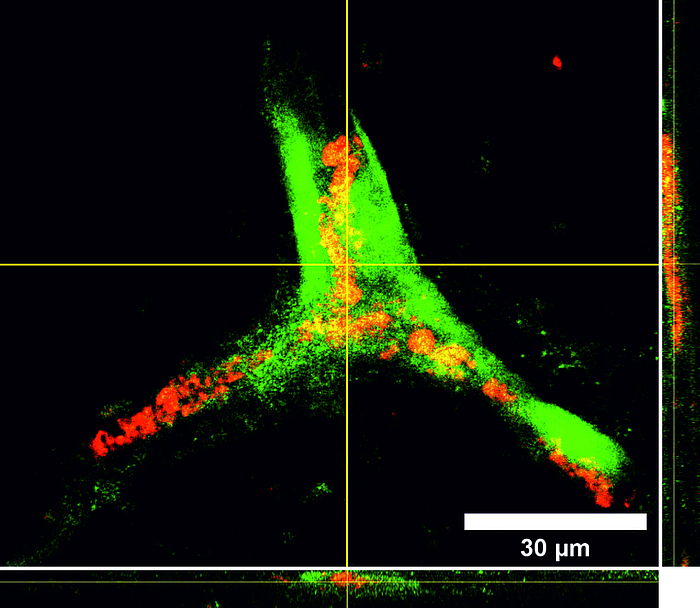
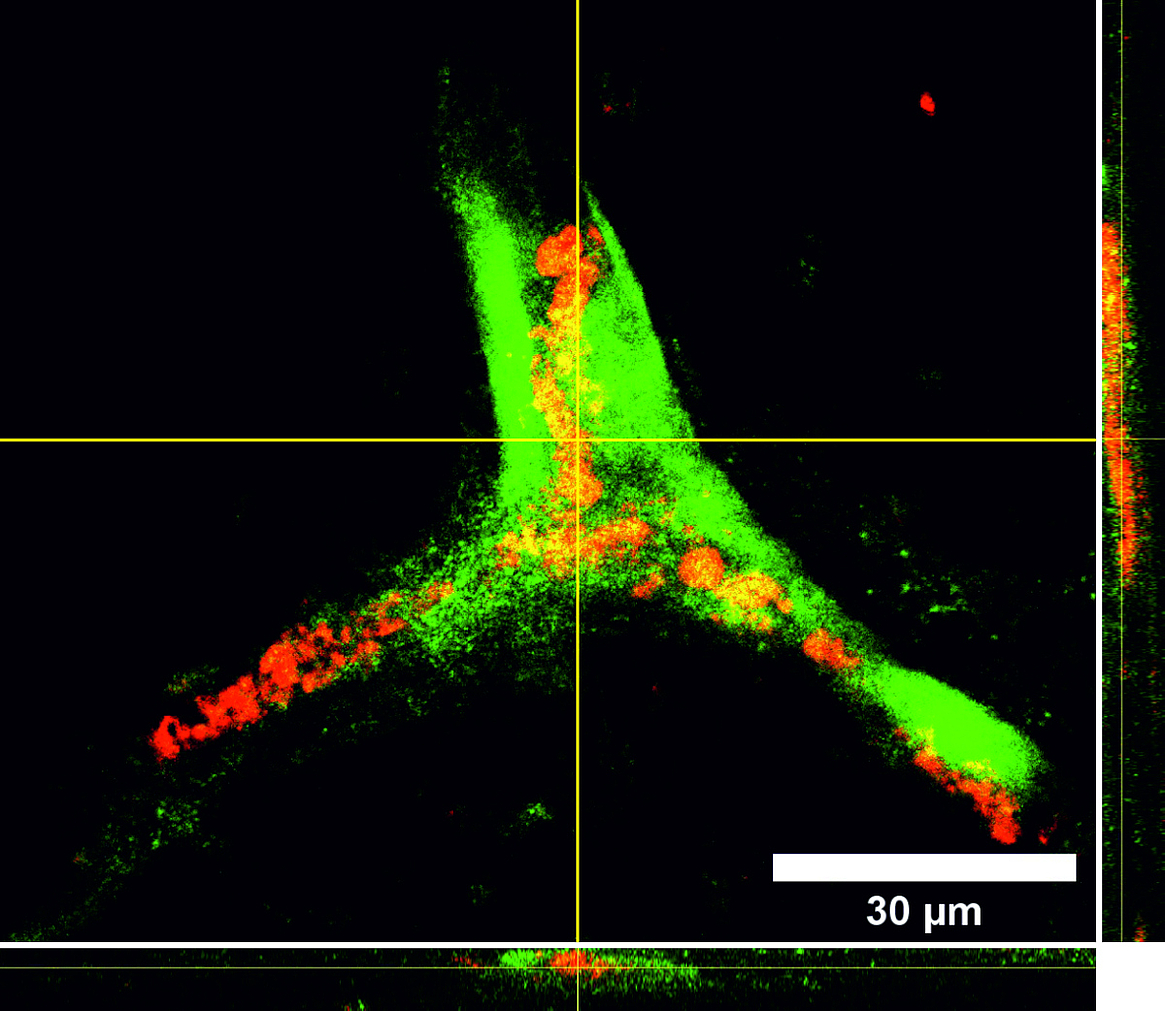
Tissue engineering is a rapidly growing sector in the field of future-oriented medicine and offers enormous potential in the development of bioartificial vascular prostheses, which are becoming increasingly important in a rapidly aging society. One of the main problems within this project is the isolation and cultivation of endothelial cells under the aspect of autologous transplantation. However, recent studies show that human mesenchymal stem cells isolated from adipose tissue of patients (hASCs, cooperation with the Department of Plastic Surgery of the MHH) have approaches for differentiation in an endothelial direction and positively influence the differentiation of immature endothelial cells into capillaries. We therefore developed a protocol for the targeted use of stem cells in coculture with endothelial cells. For the induced secretion of proangiogenic markers, the cultivation parameters hypoxia and shear stress play an important role. In parallel, we isolate endothelial progenitor cells from peripheral blood (cooperation with the blood bank of the MHH) and optimize their extracorporeal expansion and development into a confluent cell layer on scaffold materials for endothelialization. Flow cytometric analysis of specific cell subsets and selective subcultivation supports the formation of mature endothelial monolayers. In addition, we investigate the influence of different shear stress conditions (static vs. dynamic cultivation under pulsatile or laminar flow of different flow velocities) on these endothelial progenitor cells isolated from peripheral blood.
Bioprinting and scaffold development
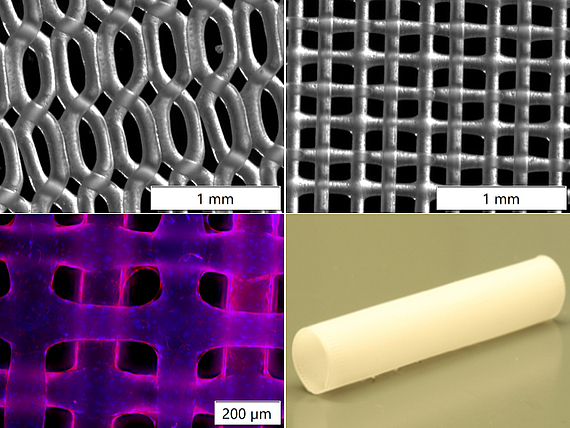
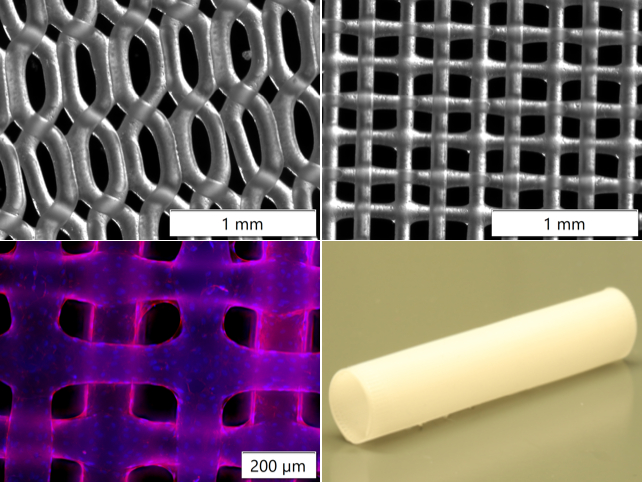

In the field of bioprinting, we are collaborating with the AG "Blume" (eNIFE) on a BioX bioprinter from Cell-Ink (Sweden) and Regen-Hu (Basel, Switzerland). Novel biodegradable polymers are tested as scaffold material and 3D-printed in fine lattice structures by 3 D bioprinting or electrospinning-writing which are coated with native proteins and seeded with endothelial cells. Here, we cooperate with Prof. Dr. M. Wilhelmi and his group working on decellularized animal arteries and performing large animal experiments. Microstructured two-component scaffolds are seeded using viscous bioinks. Isolation of progenitor cells from the bloodstream for endothelialization of the vascular prosthesis was optimized using the principle of dynamic cell cultivation, which places cells under shear stress. Porous scaffolds prepared by porogen leaching are co-cultured with mesenchymal stem cells and endothelial cells (so called HUVECS= human umbilical venous endothelial cells) and capillary structures are grown.
Bioreactor development



In cooperation with eNIFE, our research group developed a functional bioreactor for the clinical supply and cultivation of a "vascular graft" (bioartificial vascular prosthesis). In contrast to existing systems, this bioreactor also regulates the differentiation process of the growing graft and controls the cultivation conditions. For this purpose, sensor systems for pH, temperature, glucose, etc. were adapted and non-contact monitoring via ultrasound was established. The bioreactor has already been awarded with the VDI prize in 2018 and is now being optimized in line with current concepts of Medical Device Regulation. The following topics are being worked on here: Sterility tests, tightness tests, monitoring concepts by means of sensor technology, pressure monitoring i. Vgl. with Comsol-Estimierungen for near-wall shear stress during a cultivation, flow measurements by means of various tubular scaffolds, among others, from pig and horse, but also from the 3 D bio-printer
Biotesting
Another field of activity of the working group is biotesting. This involves the screening of different pharmacologically active substances with regard to their effect on proliferation behavior and their toxicity to human primary cells and cell lines. For the screening established assays (CTB, MTT, ECIS, Apo ONE) are used as well as new methods are established, which include experiment-specific questions in more detail. These include toxicity determination of modern immunosuppressants in established 3D spheroid systems. For example, our results explain the endothelium-destroying effect of the calcineurin inhibitor tacrolimus, which is observable in the clinic, even under controlled clinical conditions. Furthermore, two postdoctoral researchers and a doctoral student at the Institute of Technical Chemistry in Nordstadt are working on the development of diagnostic test systems such as live cell microarrays (Dr. R. Jonczyk, DFG), lateral flow assays for kidney transplant rejection based on sensitive antibodies and on improving diagnostics and individualised T cell therapy for polyomavirus infection after transplantation. This ERDF (European Regional Development Fund)-funded project is in cooperation with the Institute of Transfusion Medicine and Transplant Engineering, the Department of Nephrology at the MHH and the Department of Information and Kommunikation at Hochschule Hannover University of Applied Sciences and Arts.
HLA I specific T-Cell Finder after infection
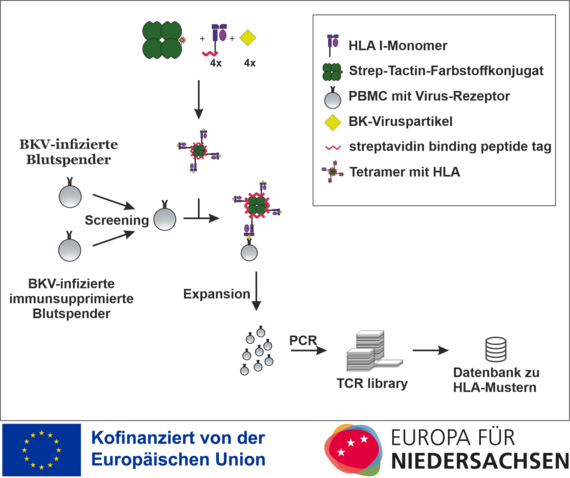
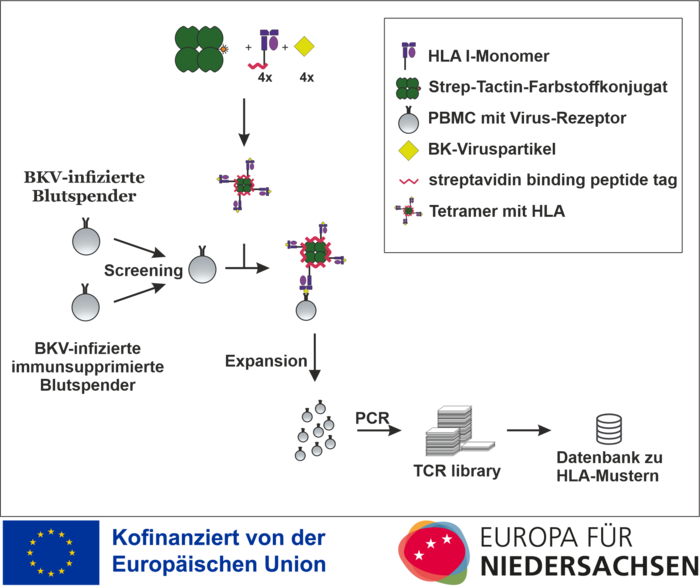
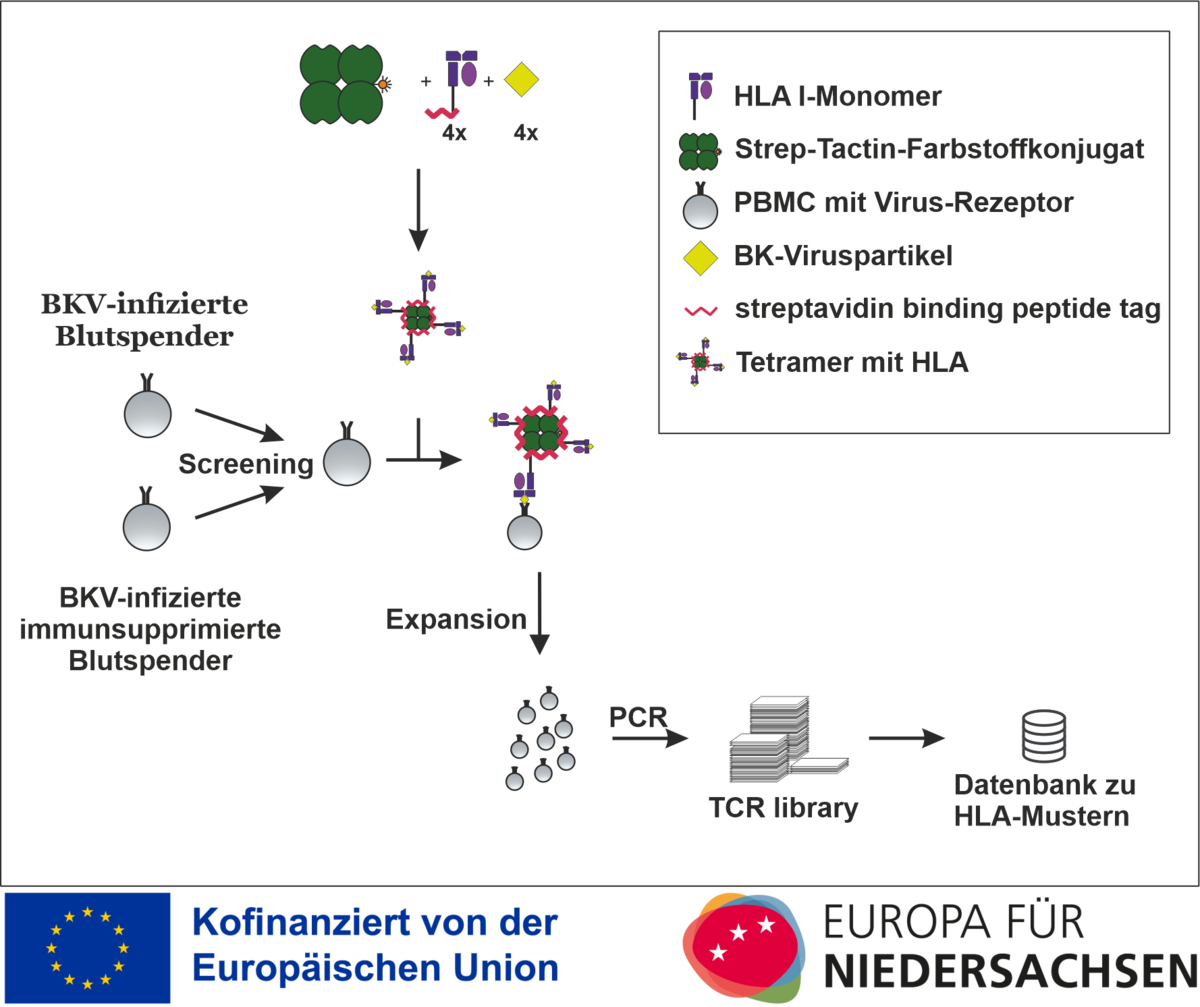
The project is funded by the European Regional Development Fund (ERDF) and the state of Lower Saxony and deals with HLA streptamers to improve diagnostics and individualised T-cell therapy for polyomavirus infection after transplantation.
Kidney transplantation as the therapy of choice for terminal kidney failure and bone marrow transplantation/stem cell transplantation are the main therapeutic pillars in the treatment of malignant haematological diseases. As a consequence, these diseases generally require the patient to receive lifelong immunosuppressive measures in order to prevent the occurrence of acute and chronic rejection reactions. However, the use of immunosuppressive therapies is associated with a significantly increased susceptibility to infection in these patients. An infection with the BK virus (BKV) can lead to chronic polyomavirus nephropathy (PVAN). For this reason, preventive screening procedures for BK viraemia are recommended. However, the reduction of immunosuppression also harbours the risk of an acute or chronic rejection reaction or graft-versus-host disease. Both PVAN and these undesirable rejection reactions, which can only be confirmed by biopsy, can lead to a deterioration in kidney function and even to the need for dialysis.
Optogenetics
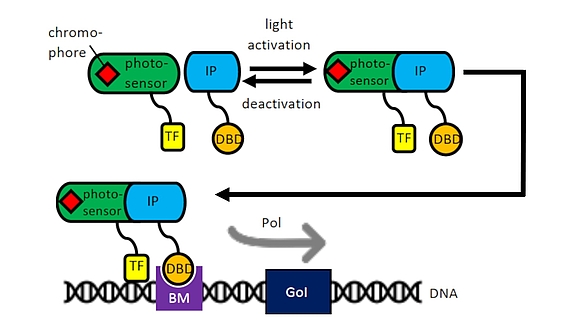
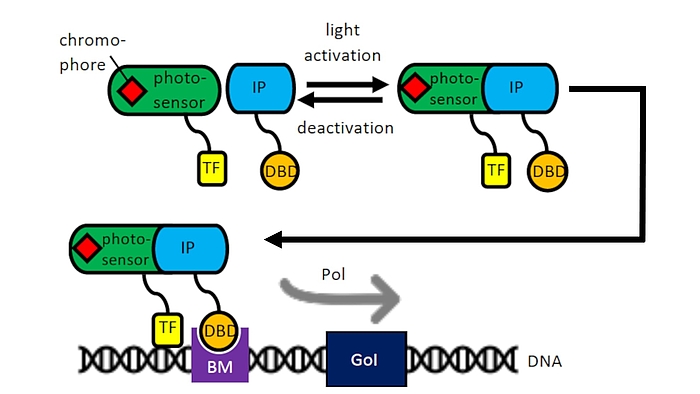
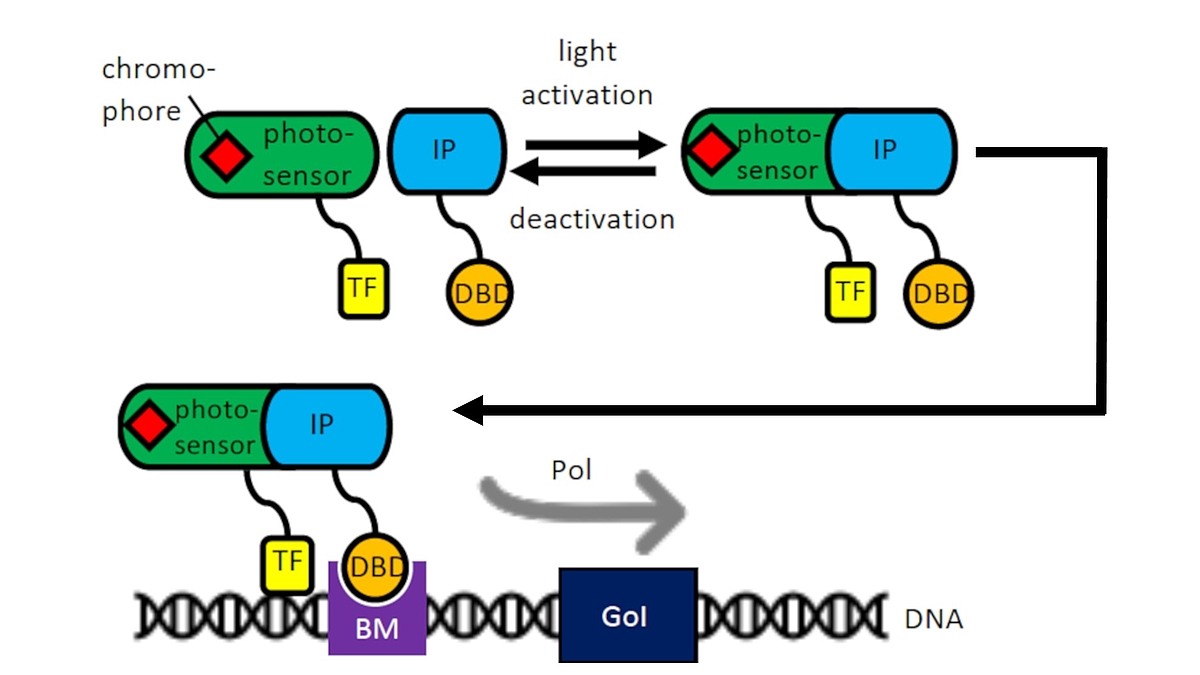
As part of the Cluster of Excellence “Hearing for All” we use our experience with mesenchymal stem cells and cell transfections for the development of optogenetically activatable cells for human therapy. In cooperation with the group of Prof. A. Warnecke, light induction by means of an optogenetic switch will be used to induce the transcription of certain pharmacologically or neuroprotectively important proteins, e.g. during the healing of cochlear implants in the context of accompanying cell therapies. Enzymes can also be specifically switched on for the activation of nanoparticles in drug delivery (cooperation with the research group of Prof. P. Behrens, Inorganic Chemistry, NIFE). An optogenetic system of the WG of Prof. W. Weber, University of Freiburg could already be successfully optimized in CHO cells for induction by laser systems of the WG Prof. A. Heisterkamp in NIFE and is currently being transferred to mesenchymal stem cells from adipose tissue or bone marrow.
Kidney Disease Patients Database Project



This project is concerned with the evaluation of a longitudinal database on patients with chronic kidney disease funded by the KfH-Stiftung Präventivmedizin e. V., which is based on data from clinical studies conducted throughout Germany. The database contains information on their disease status over time as well as laboratory values. The longitudinal axis of the project extends over 6-7 years, so that the progression of renal function decline can be observed under the influence of certain parameters. Based on the data, an Innofund-funded project in the field of health services research was obtained. In this project, a risk score for assessing the risk of becoming a dialysis patient is to be evaluated, calculated and tested on patients. These patients will be recruited in four neighboring clinics of the Knappschaft (a healthcare provider that is also an insurer) in the Essen-Bottrop area and provided with an automated discharge management system. Every Knappschaft patient admitted there is screened as part of their clinical inpatient stay. Patients who show a risk of 15% or higher for dialysis in the next 5 years are automatically referred to a nephrology specialist for further care. The efficiency of this measure will be evaluated in the project and - if successful - can be incorporated into standard and billable clinical care practice. Partners in the project are - in addition to the miners' hospitals and the nephrologist Prof. Dr. Hollenbeck based there - Prof. Binder at the University of Freiburg (evaluator) and the IMIBE (Institute for Medical Informatics, Biometry and Epidemiology) in Essen.
Contact


30167 Hannover




