

 ©
Harry Köster/ harry-koester.de
©
Harry Köster/ harry-koester.de
The research group is dedicated to exploring innovative strategies for in vitro expansion and the targeted optimization of culture conditions for human and mammalian cells, aiming to enhance their applications in research, medicine, and biotechnology. In addition to conventional cultivation techniques, such as static two-dimensional (2D) cell culture, three-dimensional (3D) cell culture systems are also established and characterized.
Human MSCs, cell lines, and primary cells are cultured as cell aggregates, on scaffolds, and in hydrogels under physiologically relevant, in vivo-like conditions. To enable the development of complex in vitro 3D systems, bioinks and bioprinting technologies are employed to generate physiologically relevant 3D models for drug screening.
Furthermore, the group investigates the effects of various in vitro gradients (e.g., oxygen concentration, stiffness, growth factors) on cellular behavior in hydrogels. This test system aims to reliably determine the combined influence of multiple factors to define optimal culture conditions for specific applications. Additionally, various cell types have been modified with genetically encoded sensors (e.g., hypoxia, apoptosis, YAP/TAZ, glucose) to analyze their response in different 3D culture systems (cell aggregates, hydrogels, and scaffolds). For this purpose, specially designed microscopy objectives and AI-supported algorithms for cell and signal recognition are utilized.
In collaboration with Cultimate Foods GmbH, we are also developing bioprocesses for cultivated culinary fat production. Here, stem cells isolated from cattle and pigs are ex vivo expanded and differentiated into adipocytes to generate fat, which is incorporated into plant-based meat alternatives to enhance flavor and nutritional properties.
AI-assisted monitoring and analysis of 3D cell cultures


 ©
Harry Köster/ harry-koester.de
©
Harry Köster/ harry-koester.de
In collaboration with Sartorius AG , AG Lindner and the Yoav Shechtman Lab at Technion, we are developing innovative methods for precise and efficient cell localization in 3D hydrogel-based constructs. Using standard and Extended Depth-of-Field (EDOF) objectives, combined with AI-assisted algorithms, we enhance the rapid and accurate detection of cells in brightfield Z-stack images. Together with widefield fluorescence imaging and the IncuCyte® Imaging System, this approach enables the analysis of fluorescent sensor cells, allowing real-time observation of local sensor signals and the generation of spatial heatmaps of cell responses in 3D cultures.
Gradient hydrogels
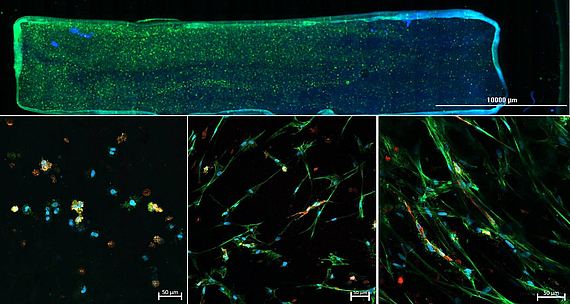
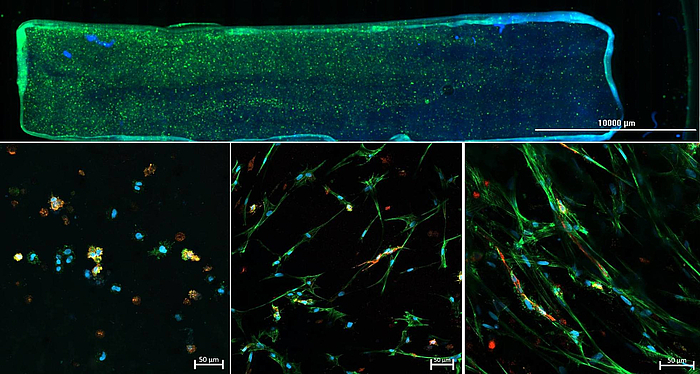
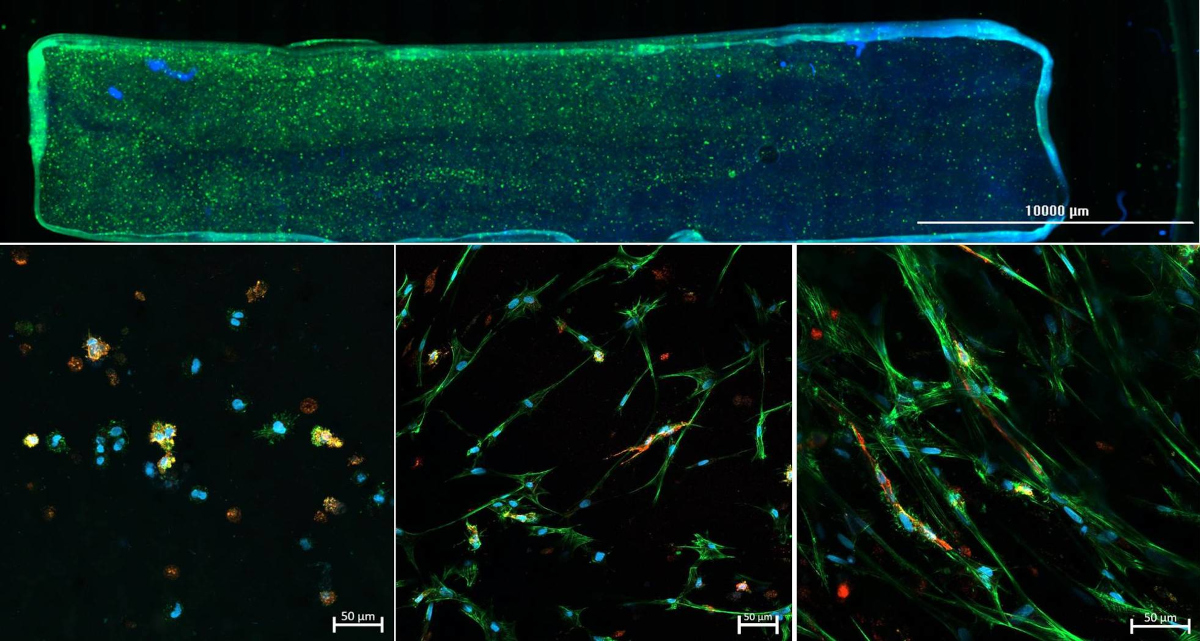
As part of the DFG-funded project "3D Two Gradient Systems for Functional Cell Testing" (398007461), we are developing hydrogel-based in vitro gradients. The aim of the present research project is the development of a 3D cell culture system where multiple-gradient hydrogels can be created in order to obtain complex, but precisely defined in vitro microenvironments. Through variation of the hydrogel composition, mechanical gradients can be realized in the culture model, which allow a systematic investigation of the influence of microenvironmental conditions. Orthogonally, an oxygen concentration gradient is established, so that combinations of hydrogel composition and different oxygen concentrations can be tested within one experiment. With the help of this novel test system, it should be possible to quickly and reliably determine the combined influence of various factors on cell behavior, in order to find optimal culture conditions for specific applications.
Integration of genetically encoded biosensors for real-time monitoring in 3D


 ©
Harry Köster/ harry-koester.de
©
Harry Köster/ harry-koester.de
As part of the interdisciplinary research consortium Matrix Evolution, funded by the Lower Saxony Ministry of Science and Culture, the Lavrentieva Group leads the subproject "MatrixSense" . Our research focuses on the integration of genetically encoded biosensors for real-time monitoring in relevant cell types, and the characterization of reporter cells in 2D and 3D cell culture systems. Additionally, we develop strategies for the real-time online evaluation of spatiotemporal cellular responses at the single-cell level within chirarchically structured 3D matrices. To achieve this, we integrate hypoxia, apoptosis, YAP, actin, pH, and vinculin sensors into MSCs and endothelial cells.
For monitoring cellular signaling pathways, we utilize an innovative live-cell analysis system with high-resolution fluorescence imaging, enabling real-time observation and quantification of complex biological changes.
In vitro Cultivated Fat


 ©
Harry Köster/ harry-koester.de
©
Harry Köster/ harry-koester.de
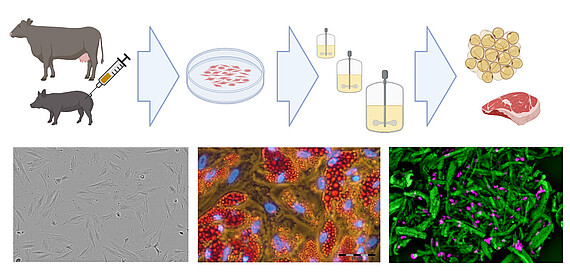
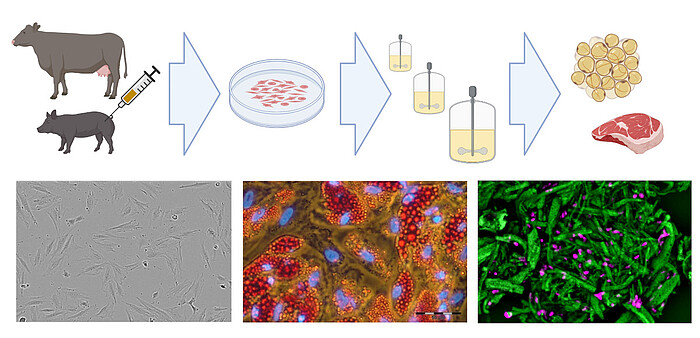
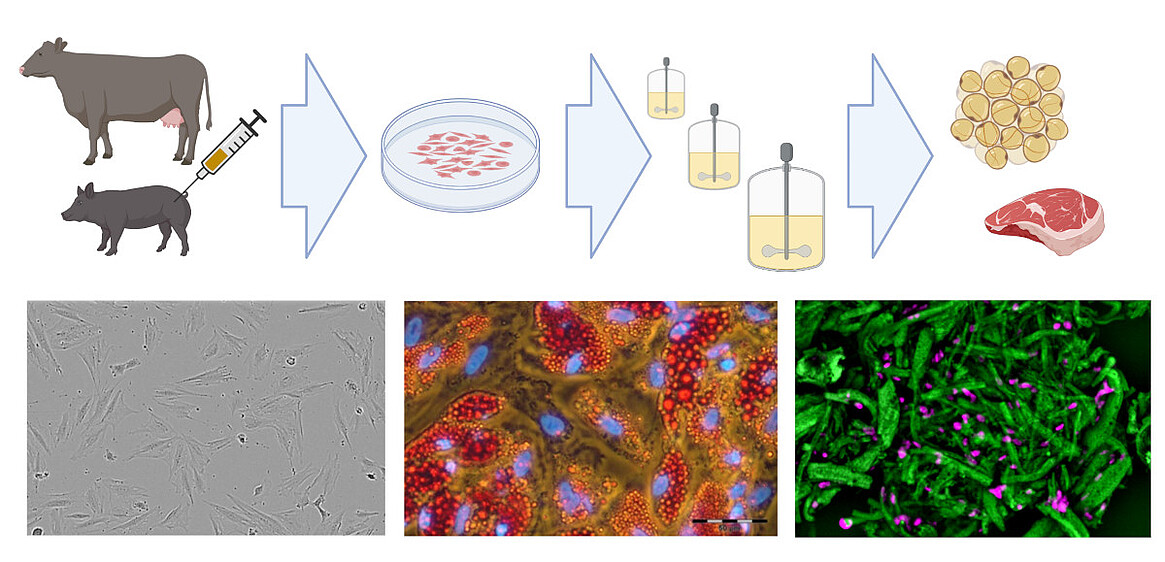
Existing know-how in mammalian cell cultivation (including cultivation of mesenchymal stem cells) can also be transferred to the rapidly growing field of novel foods, especially to cellular agriculture. Here, cells isolated from agronomically important species are expanded and differentiated ex vivo to produce either cell biomass for unstructured meat or cells growing in scaffolds for structured meat analogs. In cooperation with the start-up Cultimate Foods from Berlin/Hannover, our working group is developing bioprocesses for the isolation, expansion and differentiation of anchorage-dependent porcine and bovine cells for the production of cell-cultured fat. The manufacturing of cultured fat for food is challenging as it requires a cost-effective process that includes only food-grade cell culture components. Nevertheless, there are already first promising results that give the technology great hope.
Contact


30167 Hannover




